OSU forms plan to prepare for COVID-19 vaccine, related concerns
January 4, 2021
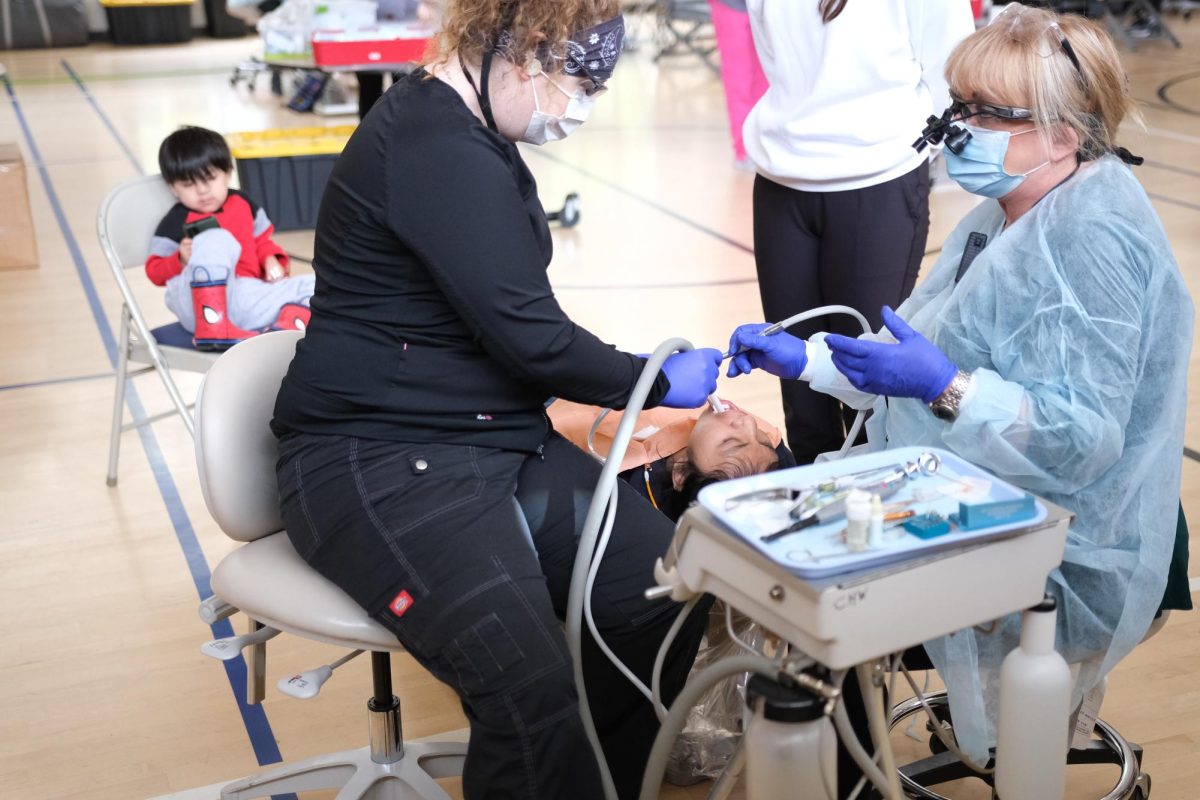
Following two COVID-19 vaccines—Pfizer and Moderna—being approved by the Food and Drug Administration in December, Oregon State University has begun creating a plan to deal with these vaccines at both campus-wide and statewide levels.
While these vaccines are currently being administered to people around the United States, a priority is being placed on health care providers first, followed by staff and residents in long-term care facilities as well as those with compromised immune systems who are most at risk for the virus. It may be months before most college students see any sort of COVID-19 vaccine.
Even so, it’s only a matter of time before vaccines come to OSU’s campus, but the next few months will be crucial for studying the vaccines as well as any side effects they may have.
Chunhuei Chi, professor of the Global Health Program and Health Management and Policy program and director of the Center for Global Health in the College of Public Health and Human Sciences, said it is important to begin developing a plan when it comes to the vaccine, but not finalize it until they have time to observe three main issues throughout the process.
According to Chi, the issues they want to observe are “how well the vaccine is performing at the population level in terms of immunity and safety; how well is the distribution channel and infrastructure of vaccination in meeting the population needs; and how well is the public accepting the vaccination and in follow-up for the booster shots.”
These three questions about the vaccine need to be considered, and there are multiple groups of people who according to the Centers for Disease Control and Prevention should be vaccinated before relatively healthy college-age students can be. Despite this, OSU is already doing its part to help support and distribute the vaccine throughout the state of Oregon.
“It is OSU’s understanding that vaccines will be distributed by region as part of a statewide plan guided by the Oregon Health Authority,” Dan Larson, the vice president for student affairs and OSU coronavirus coordinator said. “OSU will provide support within regions as requested by the state of Oregon and the regional county health providers and vaccination centers. OSU will play an important supporting role in areas where OSU has campus or university operations, including OSU Extension Offices.”
According to Larson, Oregon Gov. Kate Brown’s office is organizing an advisory group to direct distribution of the vaccine among essential works, those who are considered high risk and to reduce health disparities for those who have historically had limited access to health care.
A Gallup Poll published on Dec. 8, found about 63% of Americans would be willing to receive an FDA approved COVID-19 vaccine, which is up from September, 50% and October, 58%. However, 70% of the American population needs to be vaccinated to achieve herd immunity.
Public confidence in the process of the COVID-19 vaccine development has risen, the Pew Research Center found 75% have a fair amount of confidence in the development process compared to 65% in September. According to Chi, OSU should monitor the public’s acceptance and confidence related to the vaccines.
Based on the data from the COVID-19 vaccine trials, it has been shown to be relatively safe, causing only mild side effects in the volunteers who participated.
“I understand that there may be concerns over the safety and efficacy of a COVID-19 vaccine,” said Sunil Khanna, the Roberts & Sara Rothschild Endowed Chair in Global Health. “The FDA’s vaccine approval process is guided by science and data. Anyone who wants to see the data can find the data on the FDA Website. Based on the currently available information, the medical community is cautiously optimistic that both Pfizer and Moderna vaccines are safe and effective.”
Chi said it’s normal for the time to develop a safe and effective vaccine to gradually shorten as technology advances. Other major concerns will need to be addressed though, including the long-term effects on sustained immunity, potential long-term side-effects, and potential interactions or allergic reactions with various diseases, health conditions and medications.
Another concern that will need to be addressed is whether the COVID-19 vaccination will be required for students and faculty.
“OSU will evaluate whether a vaccination mandate would be possible or advisable,” Larson said. “Ideally, reaching sufficient population protection will not require the use of a mandate. Consideration of such a requirement will include evaluation of many issues and will involve consulting with public health authorities and other stakeholders and understanding best practices nationally.”
While waiting for the vaccine, students and faculty are continually encouraged to continue following the COVID-19 guidelines, including wearing a mask, staying six feet apart from each other and washing their hands, until and even after the vaccine gets distributed to the university community.
“It is important for the OSU community to stay vigilant in hygiene-safe practice against COVID-19, and not lift any guard because of the availability of effective vaccines,” Chi said. “It will take several months from day-one when OSU begins to implement its vaccination policy to make OSU safe for in-person activities. One of the worst scenarios is that the OSU community lifts its hygiene-safety practices because of the vaccine-induced premature sense of security against the pandemic.”